Abstract
Background: People with hemophilia A (PwHA) face unique challenges in managing their condition, which can impact health-related quality of life. Those who are receiving optimal prophylactic treatment may be able to overcome some of these challenges. Measuring patient-reported outcomes (PROs) could help clinicians and PwHA understand the impact of prophylaxis.
Methods: This was a survey designed to assess PROs in adult PwHA receiving prophylactic treatment with either emicizumab or factor VIII (FVIII) replacement therapy. Respondents were recruited through the membership of the Hemophilia Federation of America. To facilitate descriptive comparisons between the experiences of PwHA on either of the two prophylaxis regimens, PwHA with inhibitors were not included. Survey questions focused on outcomes related to healthcare resource use (clinic and emergency department [ED] visits), out-of-pocket expenses, and employment status. Responses are reported in counts and proportions or means with standard deviations (SD).
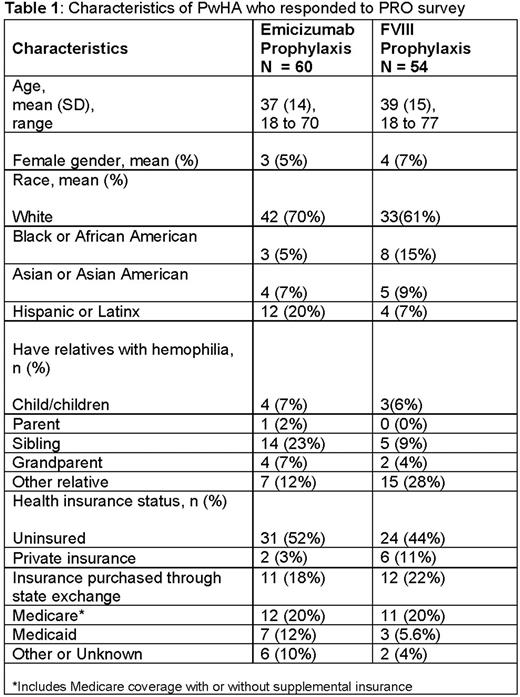
Results: A total of 114 PwHA (60 who reported taking emicizumab and 54 taking FVIII for prophylaxis) responded to the survey. Among them, 89 (78%) received their care at 38 different Hemophilia Treatment Centers across the US. Characteristics of survey respondents are reported in Table 1. Of note, among those receiving emicizumab, 51 (85%) had severe, 7 (12%) had moderate, and 2 (3%) had mild hemophilia A. Among those receiving FVIII, 39 (72%) had severe, 10 (19%) had moderate, and 5 (9%) had mild disease. When asked about healthcare resource use over the past 6 months, PwHA receiving emicizumab reported a mean (SD, range) of 1 (0.9, 0 to 5) clinic visit and 0.1 (0.4, 0 to 2) ED visit, while those receiving FVIII prophylaxis reported a mean of 1.2 (1.4, 0 to 10) clinic visits and 0 (0.2, 0 to 1) ED visits. When asked about out-of-pocket expenses, 19 (32%) of respondents receiving emicizumab and 18 (33%) of those receiving FVIII reported having none. The highest average (SD) hemophilia-related out-of-pocket expenses included lodging (emicizumab vs FVIII: $62 [98] vs $363 [1,997]), over-the-counter medication ($49 [83] vs $125 [433]), prescription medication ($24 [52] vs $58 [249]), and childcare ($27 [44] vs $35 [70]). When asked questions about employment, 22 (37%) of respondents receiving emicizumab and 27 (50%) of those receiving FVIII prophylaxis reported that hemophilia had no impact on their professional or work life. Conversely, 17 (28%) of respondents receiving emicizumab and 17 (32%) of those receiving FVIII reported changing their line of work or career due to hemophilia. Notably, 13 (22%) and 12 (22%) reported having to take a job that offered good health insurance, among those receiving emicizumab and FVIII prophylaxis, respectively.
Conclusions: In this survey PwHA receiving prophylactic treatment reported low healthcare resource use, variable out-of-pocket expenses, and a spectrum of hemophilia-related impacts on employment. This study highlights the heterogeneity of experiences among PwHA and potential opportunities for optimization of the patient experience with prophylactic treatment.
Disclosures
Chupka:Hemophilia Federation of America: Current Employment. Decker-Palmer:Genentech, Inc.: Current Employment; Roche Holding: Current equity holder in private company, Current holder of stock options in a privately-held company. Lin:Genentech, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Martin:Cerner Enviza: Current Employment; Roche/Genentech: Research Funding. Modi:Cerner Enviza: Current Employment; Roche/Genentech: Research Funding. Bates:Genentech/Roche: Research Funding; Cerner Enviza: Current Employment. Thomson:Cerner Enviza: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal